

It is antiferromagnetic with a Néel temperature of 475 K. However, ferrochrome as a starting material requires a prior separation of the iron.Įxtremely pure chromium is produced by further purification steps using the van-Arkel-de-Boer process.įerrochrome is produced by reducing chromite in an electric arc furnace at 2800 ° C.Ĭhromium is a silver-white, corrosion- and tarnish-resistant hard metal that is tough, malleable and malleable in its original state. Corresponding solutions are prepared by dissolving chromium (III) oxide or ferrochrome in sulfuric acid. Purer chromium is made by electrodepositioning the Cr 3+Ions from sulfuric acid solution shown. This is followed by the aluminothermic reduction of the chromium (III) oxide to chromium:Ĭhromium cannot be obtained from oxidic ores by reduction with coal, since this creates chromium carbide. A subsequent reduction with carbon gives chromium (III) oxide: The sodium dichromate crystallizes on cooling as a dihydrate from the solution. The sodium chromate is extracted with hot water and converted into dichromate with sulfuric acid: In the second step, an oxidizing digestion takes place at approx. The extracted chromite ore is freed from the dead rock. The compounds of chromium have many different colors and are often used as pigments in paints and varnishes. It belongs to the transition metals, in the periodic table it is in the 6. Χρῶμα chrṓma, color ') is a chemical element with the element symbol Cr and the atomic number 24. The electronic configuration of Chromium will be 1s2 2s2 2p6 3s2 3p6 3d5 4s1.Chromium (alt. How do you write the electron configuration for Chromium? The electronic configuration of Chromium will be 1s2 2s2 2p6 3s2 3p6 3d5 4s1.
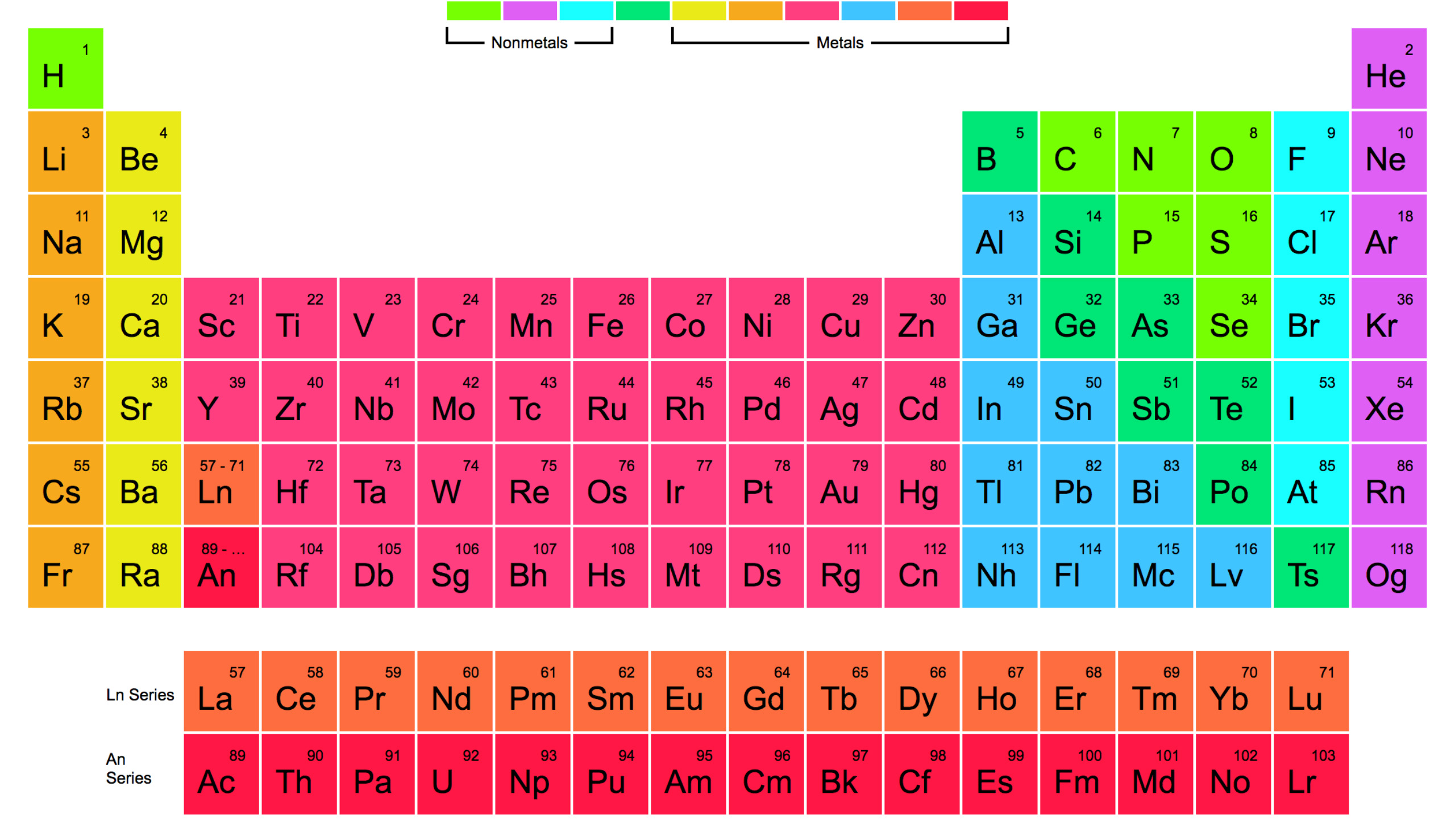
What is the electronic configuration of Chromium 24? What is the boiling Point of Chromium in Kelvin?īoiling Point of Chromium in Kelvin is 2944 K. Melting Point of Chromium in Kelvin is 2180 K. What is the melting Point of Chromium in Kelvin? What is the boiling Point of Chromium?īoiling Point of Chromium is 2944 K. Chromium has 24 electrons out of which 6 valence electrons are present in the 3d5 4s1 outer orbitals of atom. How many valence electrons does a Chromium atom have?Ĭhromium has 6 valence electrons. The element Chromium was discovered by N. What is the color of Chromium?Ĭhromium is of Silver color. It is located in group 6 and period 4 in the modern periodic table. Chromium is the 24 element on the periodic table. What is the position of Chromium in the Periodic Table?Ĭhromium is a chemical element with the symbol Cr and atomic number 24. Chromium is a chemical element with symbol Cr and atomic number 24. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. The abbreviated electronic configuration of Chromium is 3d5 4s1. What is the abbreviated electronic configuration of Chromium? The electronic configuration of Chromium is 1s2 2s2 2p6 3s2 3p6 3d5 4s1.
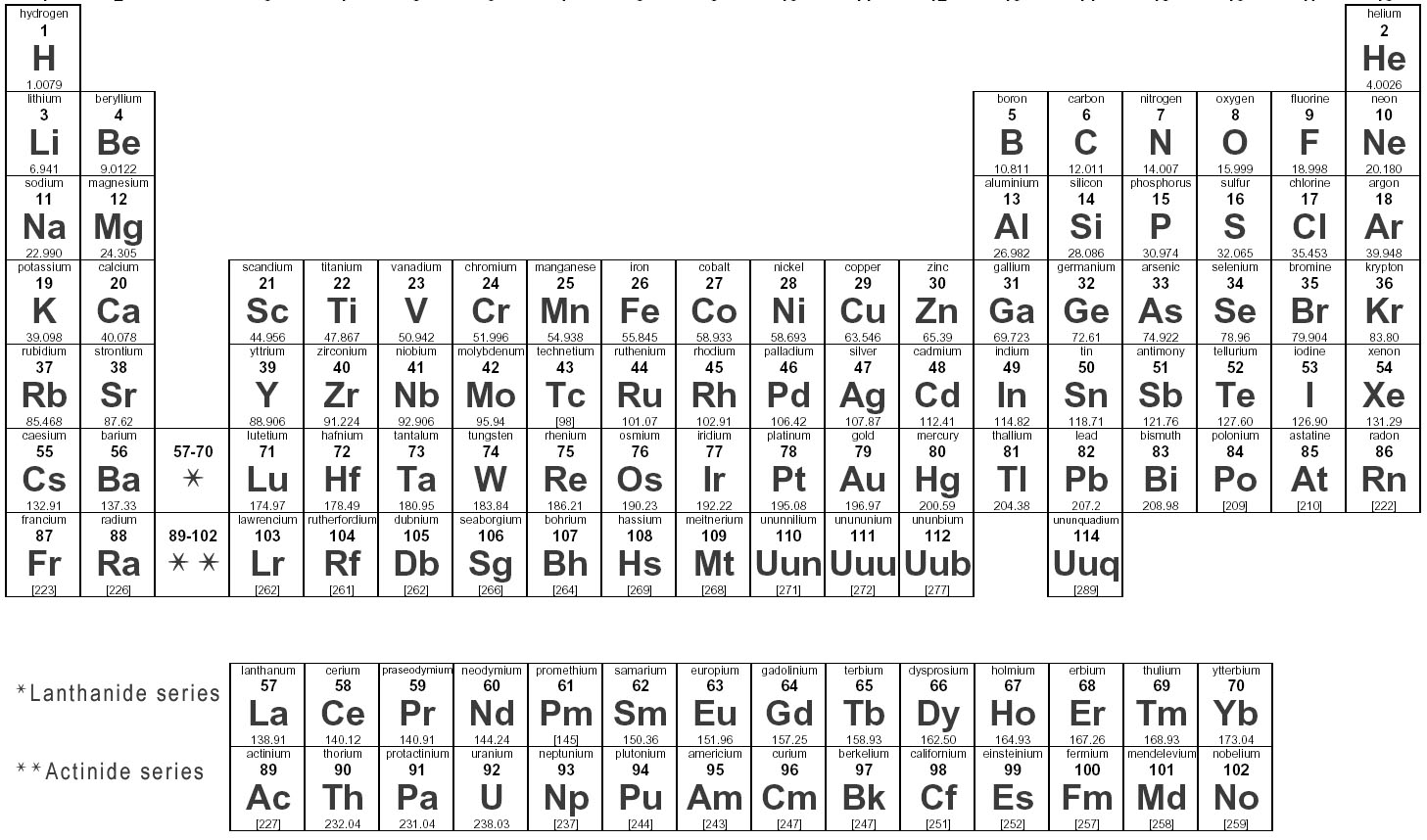
What is the electronic configuration of Chromium? Optical Properties of Chromium Refractive IndexĪcoustic Properties of Chromium Speed of SoundĬhromium Thermal Properties - Enthalpies and thermodynamics Refer to table below for the Electrical properties ofChromium Electrical ConductivityĬhromium Heat and Conduction Properties Thermal ConductivityĬhromium Magnetic Properties Magnetic Type Hardness of Chromium - Tests to Measure of Hardness of Element Mohs HardnessĬhromium is Conductor of electricity. Refer to below table for Chromium Physical Properties Densityħ.14 g/cm3(when liquid at m.p density is $6.3 g/cm3)


 0 kommentar(er)
0 kommentar(er)
